New Reproductive Frontier: Skin Cells Reprogrammed to Create Fertilizable Human Eggs
Written byTimes Magazine
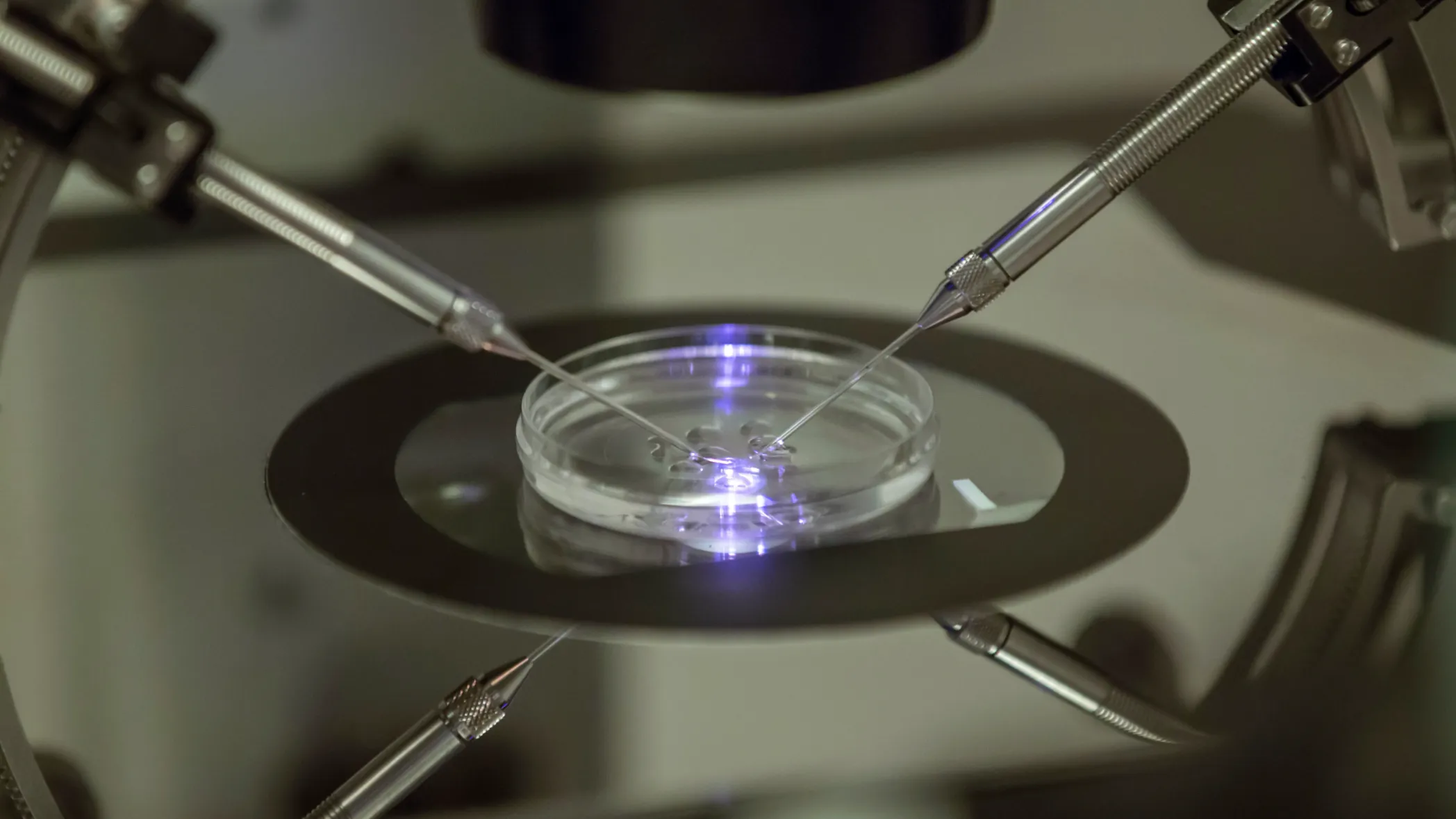
In a potentially transformative leap for reproductive medicine, a team of US scientists has successfully created early stage human embryos by utilizing DNA extracted from adult human skin cells and subsequently fertilizing them with sperm. This pioneering research, published in the journal Nature Communications, marks a significant step forward in the field of in vitro gametogenesis (IVG), the technology aiming to generate eggs and sperm outside the body.
The method, developed by researchers at the Oregon Health and Science University, involved a complex process akin to the first step in cloning, known as Somatic Cell Nuclear Transfer (SCNT). The scientists first took a common skin cell, specifically a fibroblast, and removed its nucleus, which contains the full complement of 46 chromosomes. This nucleus was then transferred into a donor egg cell from which its own nucleus had been removed. However, the skin cell nucleus contained a double set of chromosomes, whereas a normal egg requires only a single set of 23 chromosomes.
The breakthrough came with the team's ability to manipulate this reconstructed egg to expel half of its chromosomes, mimicking the natural process of meiosis which halves the genetic material to form a gamete. The researchers termed this new technique "mitomeiosis." Once the chromosomes were halved, the egg was fertilized with sperm using In Vitro Fertilization (IVF), resulting in the creation of early stage human embryos.
While the achievement is hailed as a monumental proof of concept, the technology is still in its infancy. Of the 82 eggs created, less than nine percent developed into an embryo to the blastocyst stage after six days, and none were viable due to numerous genetic abnormalities. The random nature of the chromosome halving process, which often resulted in an incorrect number or mix of chromosomes, remains the primary technical hurdle. Experts caution that this method is likely at least a decade away from being perfected for clinical application.
The long term implications of this research are profound. If the safety and efficiency issues are resolved, IVG could revolutionize fertility treatment, offering hope to millions of infertile individuals, including women who lack eggs due to age or disease. Crucially, the technique also opens the door for same sex couples to potentially have a child genetically related to both partners. However, such a groundbreaking advance immediately raises complex ethical and societal questions regarding the boundaries of human reproduction and the moral status of these lab created embryos, underscoring the urgent need for robust regulatory frameworks.